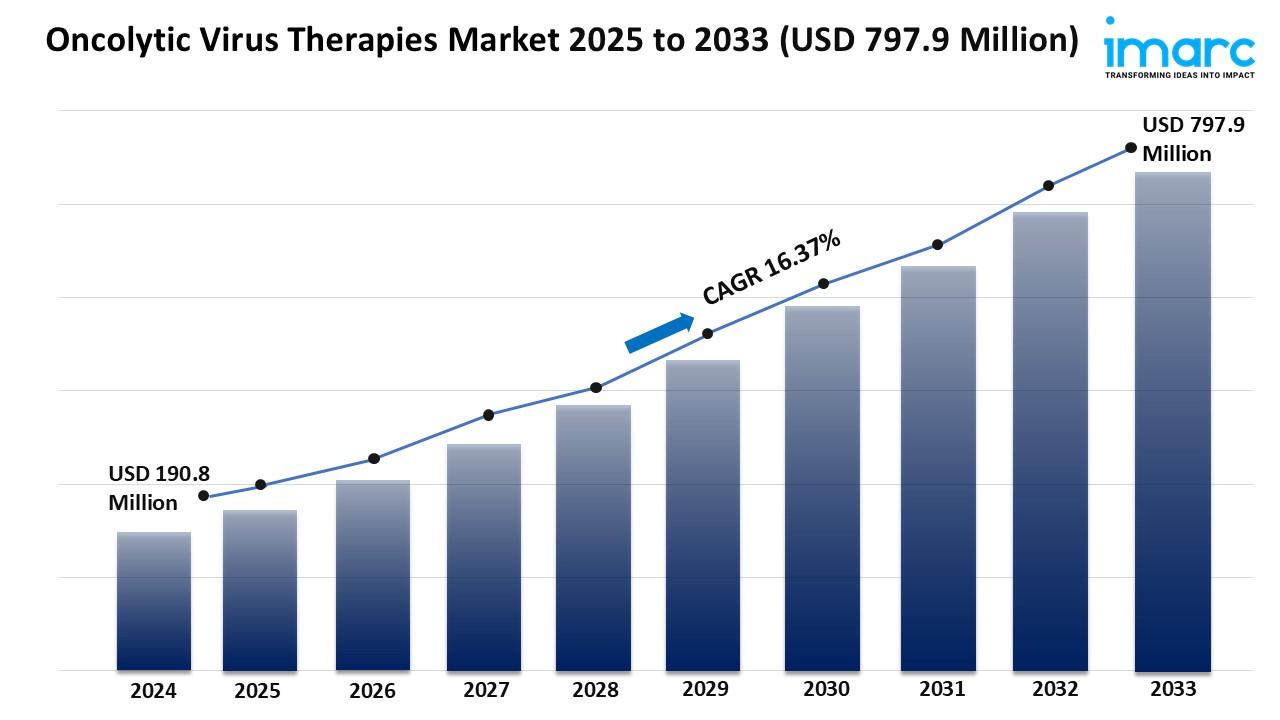
Market Overview:
The oncolytic virus therapies market is experiencing rapid growth, driven by escalating global cancer prevalence, breakthroughs in viral engineering and genetic modification, and favorable regulatory frameworks and fast-track designations. According to IMARC Group's latest research publication, "Oncolytic Virus Therapies Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global oncolytic virus therapies market size reached USD 190.8 Million in 2024. Looking forward, IMARC Group expects the market to reach USD 797.9 Million by 2033, exhibiting a growth rate (CAGR) of 16.37% during 2025-2033.
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
Download a sample PDF of this report: https://www.imarcgroup.com/oncolytic-virus-therapies-market/requestsample
Our report includes:
- Market Dynamics
- Market Trends and Market Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Growth Factors in the Oncolytic Virus Therapies Market
- Escalating Global Cancer Prevalence
The primary driver for the expansion of the oncolytic virus therapies market is the rising global incidence of cancer, which has created an urgent demand for novel treatment modalities. According to data from the World Health Organization, the global burden of cancer is expected to increase to approximately 35 million new annual diagnoses by 2050, representing a 77% surge from the 20 million cases reported in 2022. This demographic shift, largely fueled by aging populations and environmental factors, has rendered traditional therapies insufficient for many patients, particularly those with refractory or metastatic solid tumors. Consequently, healthcare systems are increasingly prioritizing the development of oncolytic viruses, which offer a unique dual mechanism of action: the direct lysis of malignant cells and the subsequent induction of a systemic anti-tumor immune response. This growing patient pool ensures a consistent and expanding addressable market for viral-based interventions.
- Breakthroughs in Viral Engineering and Genetic Modification
Significant technological advancements in synthetic biology and viral vector engineering are fundamentally transforming the efficacy and safety profiles of oncolytic therapies. Modern research has moved beyond naturally occurring viruses to focus on sophisticated, genetically modified platforms such as Herpes Simplex Virus, Adenovirus, and Vaccinia Virus. Recent industry data indicates that approximately 55% of the current therapeutic pipeline consists of these genetically engineered viruses, which are designed to selectively infect tumor cells while sparing healthy tissue. Company activities, such as those by Replimune and Genelux, involve "arming" viruses with therapeutic payloads—including cytokines like GM-CSF and IL-12—to enhance their potency. These engineering feats have mitigated previous concerns regarding viral replication control and off-target effects, leading to a higher volume of candidates entering clinical phases. Currently, the Phase I segment represents the most active development stage, driven by these innovative viral constructs.
- Favorable Regulatory Frameworks and Fast-Track Designations
Government initiatives and supportive regulatory pathways are playing a crucial role in accelerating the commercialization of oncolytic virus therapies. Regulatory bodies such as the U.S. FDA and the European Medicines Agency have introduced expedited programs like the Regenerative Medicine Advanced Therapy and the Priority Medicines scheme to provide intensive guidance for promising viral treatments. Numerical insights show that approximately 22% of current oncolytic pipeline candidates have been granted fast-track or orphan drug designations, which significantly reduces the time and cost associated with clinical development. In Japan, the Sakigake Designation System facilitated the 2021 approval of DELYTACT for malignant glioma, setting a global precedent for rapid market entry of engineered viruses. These government-led incentives enhance investor confidence and encourage biopharmaceutical giants to invest heavily in the sector, ensuring that specialized therapies reach patients with high unmet medical needs more efficiently.
Key Trends in the Oncolytic Virus Therapies Market
- Synergy Through Combination Immunotherapy
A dominant trend in the current market is the shift from monotherapy to combination treatment regimens, specifically pairing oncolytic viruses with immune checkpoint inhibitors like pembrolizumab or nivolumab. This approach aims to "turn cold tumors hot" by using the virus to alter the immunosuppressive tumor microenvironment, thereby making the cancer more susceptible to the body’s immune system. Real-world applications of this trend are evident in recent clinical trials where combination therapies have demonstrated superior patient outcomes compared to either treatment alone. For instance, Genelux’s Olvi-Vec combination trials recently reported a 71% disease control rate in small-cell lung cancer patients. Currently, approximately 40% of all oncolytic virus research efforts are focused on these combination strategies, as they offer a potential solution for patients who have previously been deemed untreatable by conventional immunotherapy standards.
- Integration of Artificial Intelligence in Viral Design
The integration of Artificial Intelligence and machine learning is revolutionizing how oncolytic viruses are discovered and optimized. AI algorithms are now being utilized to analyze massive genomic datasets to predict how specific viral modifications will interact with diverse tumor profiles. This trend allows researchers to design "next-generation" viruses with improved tropism—the ability to target specific cell types—and enhanced replication speeds. For example, AI-driven platforms can simulate thousands of genetic variations to identify the most effective viral capsid modifications before moving into physical laboratory testing. This technological application has led to a significant reduction in the early-stage development timeline, with industry insights suggesting that AI-optimized candidates are reaching the clinical trial stage faster than those developed through traditional trial-and-error methods. This shift toward "digital virology" is essential for creating more precise and personalized cancer treatments.
- Transition Toward Systemic Delivery Mechanisms
Historically, most oncolytic therapies required direct intratumoral injection, which limited their use to easily accessible surface tumors. However, a major emerging trend is the transition toward systemic or intravenous delivery, which allows the virus to reach metastatic sites throughout the body. Advanced delivery technologies, such as nanoparticle encapsulation and exosome-mediated transport, are being employed to protect the virus from being neutralized by the patient's immune system before it reaches the tumor. For example, SillaJen is actively developing the SJ-600 series, an intravenous injectable oncolytic vaccine designed to be effective against a wider range of internal malignancies. Recent market insights indicate that systemic delivery research now accounts for roughly 30% of new development activities. This evolution is critical for expanding the application of oncolytic viruses to include complex cancers like breast, lung, and ovarian malignancies that were previously difficult to target.
Leading Companies Operating in the Global Oncolytic Virus Therapies Industry:
- Amgen Inc.
- Dnatrix Inc.
- Genelux Corporation
- Lokon Pharma AB
- Replimune Group Inc.
- Rigvir Ltd.
- Sorrento Therapeutics
- Takara Bio Inc. (Takara Holdings Inc.)
- Targovax
- TILT Biotherapeutics Ltd
- Transgene SA
- Vyriad
Oncolytic Virus Therapies Market Report Segmentation:
By Virus Type:
- Genetically Engineered Oncolytic Viruses
- Herpes Simplex Virus
- Adenovirus
- Vaccinia Virus
- Oncolytic Wild-Type Viruses
- Reovirus
- Newcastle Disease Virus
- Vesicular Stomatitis Virus
By Application:
- Solid Tumors
- Breast Cancer
- Prostate Cancer
- Lung Cancer
- Glioblastoma
- Melanoma
- Hematological Malignancies
- Lymphoma
- Leukemia
- Myeloma
By End Use:
- Hospitals
- Specialty Clinics
- Cancer Research Institute
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302