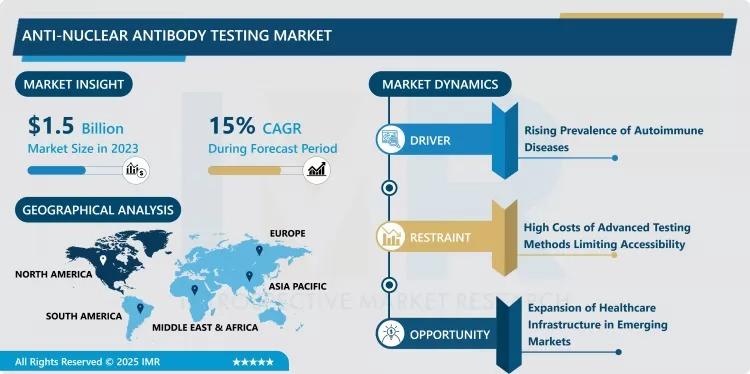
The global anti-nuclear antibody testing market is at the forefront of modern immunology, providing the primary screening tool for identifying systemic autoimmune disorders. ANA tests detect autoantibodies that target the nucleus of a patient’s own healthy cells, serving as a critical indicator for conditions such as Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis, and Scleroderma. By utilizing advanced laboratory techniques like Indirect Immunofluorescence (IIF) and Enzyme-Linked Immunosorbent Assay (ELISA), clinicians can confirm the presence of these biomarkers early in the disease cycle, significantly improving patient outcomes through timely intervention.
As the global burden of autoimmune diseases continues to rise, the role of ANA testing has expanded from specialized rheumatology clinics to broader clinical diagnostic settings. The market is currently undergoing a digital transformation, with traditional manual microscopy being replaced by automated imaging systems and AI-driven pattern recognition. These advancements address longstanding challenges in test standardization and subjective interpretation. Today, ANA testing is not only a diagnostic necessity but also a vital tool for monitoring disease progression and evaluating the efficacy of immunosuppressive therapies in a personalized medicine framework.
Market Segmentation:
The Anti-nuclear Antibody Testing Market is segmented into Product, Technique, and Application. By Product, the market is categorized into Reagents & Assay Kits, Systems, and Software & Services. By Technique, the market is categorized into ELISA, Immunofluorescence Assay, and Multiplex Assay. By Application, the market is categorized into Rheumatoid Arthritis, Systemic Lupus Erythematosus, Sjögren's Syndrome, Scleroderma, and Others.
Growth Driver:
Surging Global Prevalence of Autoimmune Disorders: The primary catalyst for the Anti-nuclear Antibody Testing Market is the alarming rise in the incidence of autoimmune diseases worldwide. Environmental triggers, lifestyle changes, and an aging global population have contributed to a significant increase in cases of lupus and rheumatoid arthritis. This epidemiological shift has led to a proportional surge in ANA test requisitions as healthcare providers prioritize early screening and accurate differential diagnosis. With approximately 5% to 10% of the global population now affected by various autoimmune conditions, the consistent demand for high-sensitivity diagnostic kits remains a key market driver.
Market Opportunity:
Integration of Artificial Intelligence and Automated Digital Pathology: A substantial opportunity exists in the development of AI-powered interpretation software for Indirect Immunofluorescence (IIF) testing. Traditionally, IIF results required highly skilled technicians to manually identify staining patterns under a microscope, leading to inter-laboratory variability. The emergence of automated digital platforms that use machine learning to classify fluorescence patterns offers superior standardization and efficiency. Companies that innovate in "Digital IFA" and cloud-based result sharing are well-positioned to capture the growing demand from high-volume diagnostic laboratories seeking to minimize human error and reduce turnaround times.
Detailed Segmentation:
The Anti-nuclear Antibody Testing Market is segmented on the basis of Product, Technique, and Application.
Product The Product segment is further classified into Reagents & Assay Kits, Systems, and Software & Services. Among these, the Reagents & Assay Kits sub-segment accounted for the highest market share in 2024. This dominance is attributed to the high volume of consumables required for routine autoimmune screening and the recurring nature of reagent purchases. As clinical laboratories process thousands of samples monthly, the constant replenishment of ready-to-use assay kits for ELISA and IFA platforms ensures a stable and dominant revenue stream. Technological improvements in reagent sensitivity and the inclusion of broader antigen targets have further solidified this segment's leading position.
Technique The Technique segment is further classified into ELISA, Immunofluorescence Assay, and Multiplex Assay. Among these, the Immunofluorescence Assay sub-segment accounted for the highest market share in 2024. IFA remains the "gold standard" for ANA screening due to its high sensitivity and ability to provide visual staining patterns that suggest specific autoimmune pathologies. While ELISA is favored for its quantitative results and cost-effectiveness, clinical guidelines continue to recommend IFA as the preferred primary screening method. The recent introduction of automated IFA readers has rejuvenated this segment by addressing its historical drawback of subjective manual interpretation.
Some of The Leading/Active Market Players Are-
· Thermo Fisher Scientific Inc. (USA)
· Bio-Rad Laboratories, Inc. (USA)
· Abbott Laboratories (USA)
· EUROIMMUN Medizinische Labordiagnostika AG (Germany)
· Werfen (Inova Diagnostics) (Spain)
· ZEUS Scientific, Inc. (USA)
· Trinity Biotech plc (Ireland)
· F. Hoffmann-La Roche Ltd. (Switzerland)
· Erba Mannheim (Germany)
· Antibodies Incorporated (USA)
· Immuno Concepts NA Ltd. (USA)
· Merck KGaA (Germany)
· Revvity (PerkinElmer) (USA)
· QuidelOrtho Corporation (USA)
· Abcam plc (UK)
and other active players.
Key Industry Developments
News 1: In August 2024, Thermo Fisher Scientific introduced a new automated ANA testing platform featuring integrated AI-driven result interpretation. This system is specifically designed to enhance testing efficiency and accuracy in high-volume laboratory environments. The platform utilizes advanced imaging and machine learning to assist pathologists in identifying complex autoantibody patterns. By reducing manual microscopy time and standardizing result reporting, Thermo Fisher aims to help clinical laboratories meet the rising demand for autoimmune diagnostics while maintaining high clinical confidence and shorter turnaround times.
News 2: In June 2024, Abbott Laboratories announced the launch of a next-generation multiplex ANA testing kit capable of measuring over 20 distinct autoantibodies simultaneously. This launch is aimed at providing a more detailed strategy for comprehensive autoimmune disease profiling. By allowing for the simultaneous detection of markers such as anti-dsDNA, Sm, and SSA/Ro within a single run, the new kit streamlines the diagnostic workflow from screening to confirmation. This innovation supports clinicians in achieving faster differential diagnosis, particularly for patients presenting with overlapping symptoms of multiple connective tissue diseases.
Key Findings of the Study
· Reagents & Assay Kits dominate the market due to the high volume of daily screening tests.
· North America remains the largest market, while Asia-Pacific is the fastest-growing region through 2033.
· Systemic Lupus Erythematosus (SLE) is projected to be the fastest-growing application segment.
· AI-driven pattern recognition is the most significant trend transforming traditional laboratory workflows.
CTA: Download the Anti-nuclear Antibody Testing Market Sample Report Here
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the Anti-nuclear Antibody Testing Market. With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global Hormone Replacement Therapy Market industry.
📞 Contact Us
Introspective Market Research Pvt. Ltd.
Phone: +91-91753-37569
Email: sales@introspectivemarketresearch.com
Web: www.introspectivemarketresearch.com