The treatment for Hemophilia, a challenging inherited bleeding disorder, has historically involved frequent, costly intravenous injections of clotting factors. However, we are entering a new era of medicine where a one-time treatment may offer a near-cure.
Gene therapy—the process of introducing a functional copy of the deficient clotting factor gene directly into a patient’s cells—is poised to revolutionize care. This innovative approach promises long-term therapeutic effects, potentially freeing patients from the burden of traditional, lifelong management.
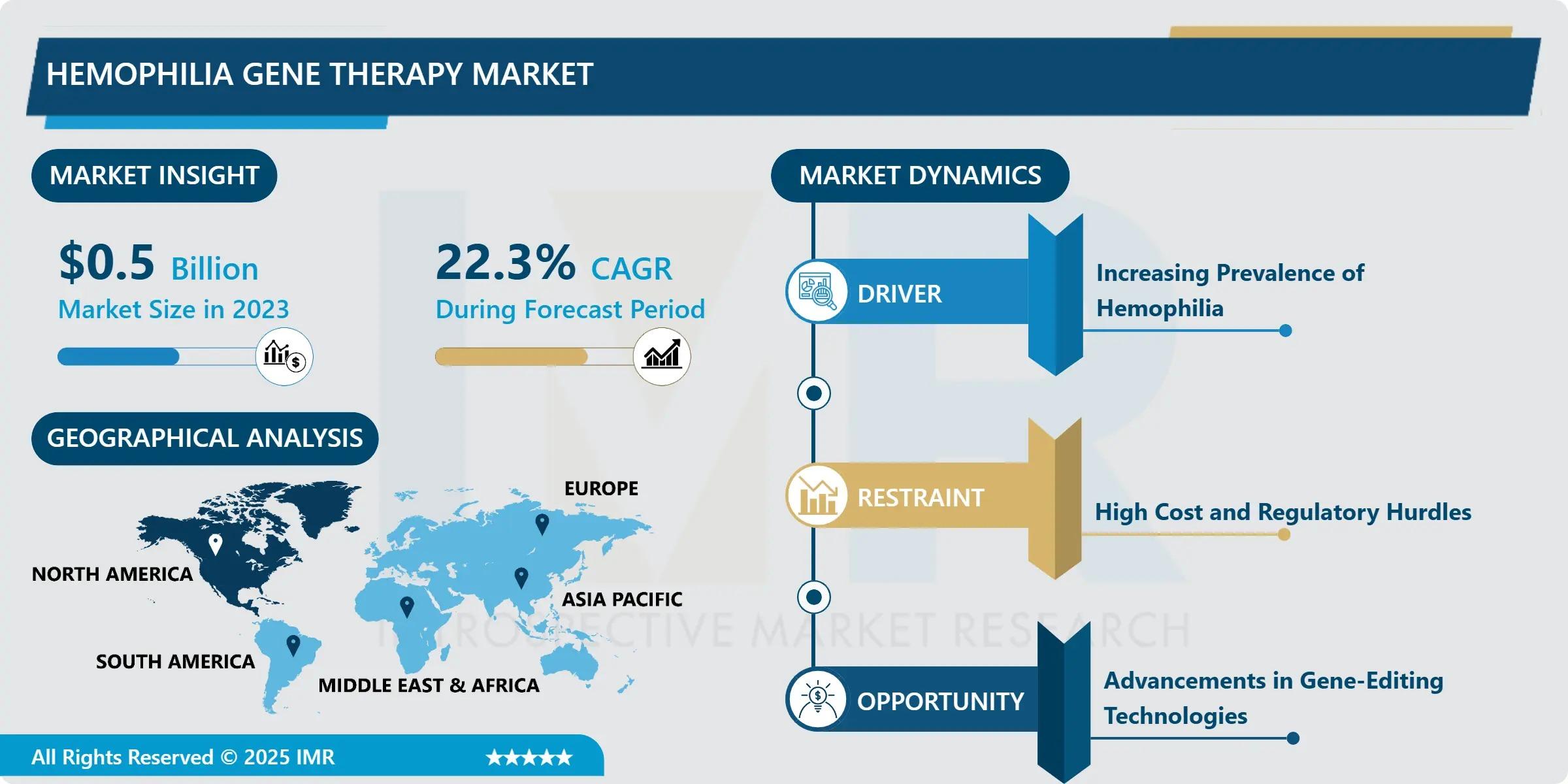
A recent market analysis confirms this groundbreaking shift, projecting massive growth for this niche segment of the biotech industry.
Market Momentum: A Staggering 22.3% CAGR
The Hemophilia Gene Therapy Market is not just growing; it's accelerating at an astonishing pace.
The market, valued at USD 0.5 Billion in 2023, is projected to reach USD 3.06 Billion by 2032, achieving a remarkable Compound Annual Growth Rate (CAGR) of 22.3% from 2024 to 2032. This exponential growth underscores the massive potential and industry confidence in gene therapy as the future of hemophilia treatment.
Key Market Catalysts Driving This Growth
Several factors are aligning to propel the market forward:
-
Addressing the Root Cause: Unlike factor replacement therapy, gene therapy directly corrects the underlying genetic defect (Factor VIII for Hemophilia A or Factor IX for Hemophilia B), offering the prospect of a permanent or long-term solution.
-
Surging Investment in R&D: The promise of a one-time cure has attracted substantial funding from government agencies, venture capitalists, and major biopharmaceutical players. These investments are accelerating the development and clinical trials of new gene-editing techniques and viral delivery systems.
-
Favorable Regulatory Landscape: Agencies like the U.S. FDA and the EMA are providing fast-track approvals and favorable regulations for these life-changing therapies, speeding up their market entry.
-
Rise of Personalized Medicine: Gene therapy is inherently a personalized approach, tailored to the patient’s genetic makeup. Advancements in technologies like CRISPR are making these treatments safer and more precise, boosting adoption rates.
Hemophilia A Dominates the Innovation Pipeline
The market is segmented by the two main types of the disease: Hemophilia A (Factor VIII deficiency) and Hemophilia B (Factor IX deficiency).
Currently, Hemophilia A is expected to dominate the market share. As the most prevalent type of the disorder, it has been the focus of extensive research, leading to multiple gene therapy products reaching advanced clinical stages and receiving accelerated market approvals in key regions.
In terms of delivery, the Hospitals segment remains the largest end-user. Gene therapy requires specialized infrastructure, highly trained personnel, and rigorous safety protocols—all of which are best provided within the secure environment of major hospital systems.
North America Leads the Genetic Revolution
Regionally, North America (especially the U.S.) commands the largest share of the market. This dominance is attributed to a combination of high hemophilia prevalence, advanced healthcare infrastructure, significant research and development spending, and the pioneering status of its regulatory environment regarding gene therapy approvals.
However, as treatments become more accessible and awareness grows globally, other regions are expected to contribute significantly to market expansion.
The Active Players in the Gene Therapy Race
The market is highly competitive, featuring both large pharmaceutical giants and specialist biotech innovators. Key companies actively pushing the boundaries of this field include:
-
BioMarin Pharmaceutical Inc.
-
CSL Behring
-
Novartis International AG
-
Pfizer Inc.
-
Takeda Pharmaceutical Company
As clinical data matures and more therapies gain approval, the Hemophilia Gene Therapy Market is set to transition from a nascent, high-potential segment into a dominant force, redefining patient care and offering hope for a life free from the constraints of this chronic condition.