"Regional Overview of Executive Summary Breakthrough Therapy (BT) Designation Market by Size and Share
The global breakthrough therapy (BT) designation market size was valued at USD 118.10 billion in 2024 and is projected to reach USD 551.69 billion by 2032, with a CAGR of 21.25% during the forecast period of 2025 to 2032.
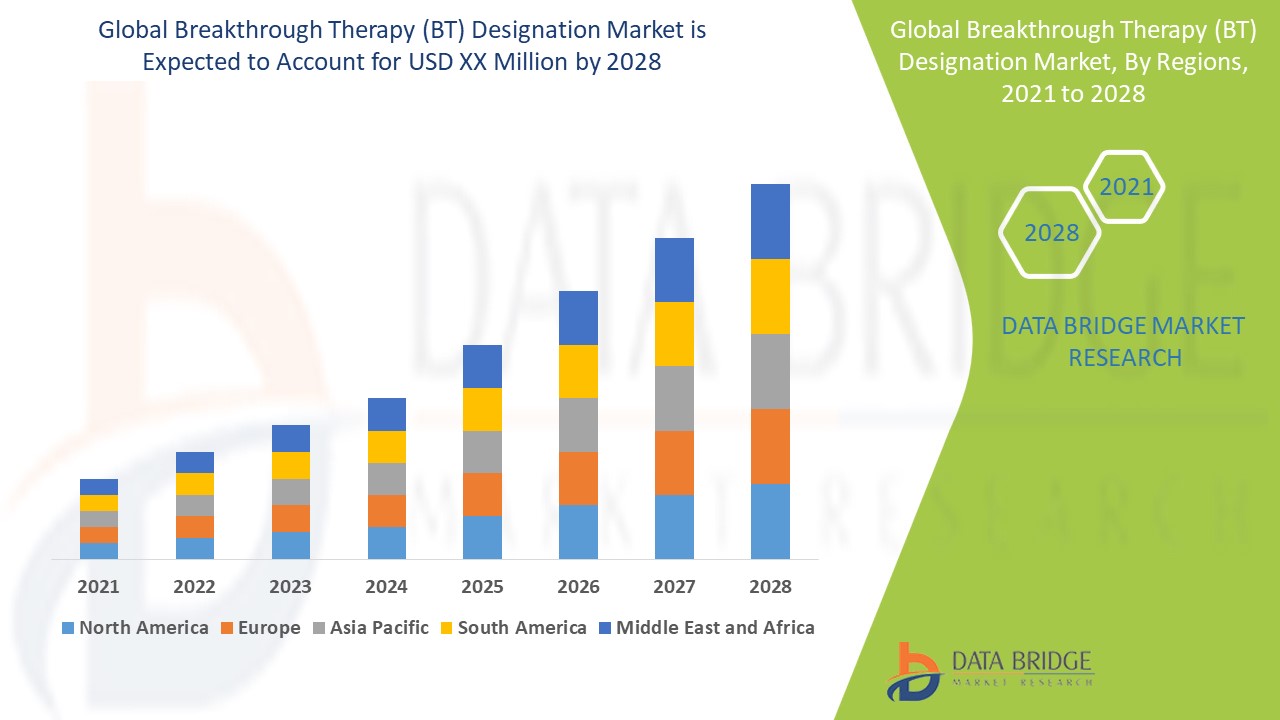
With the superior Breakthrough Therapy (BT) Designation Market report, get knowledge about the industry which explains what market definition, classifications, applications, engagements and market trends are. This report reveals the general market conditions, market trends, customer preferences, key players, current and future opportunities, geographical analysis and many other parameters that help drive the business into the right direction. The parameters of winning Breakthrough Therapy (BT) Designation Market research report range from industry outlook, market analysis, currency and pricing, value chain analysis, market overview, premium insights, key insights to the company profile of the key market players.
Besides, with the help of an influential Breakthrough Therapy (BT) Designation Market report, businesses can make out the reaction of the consumers to an already existing product in the market. This Breakthrough Therapy (BT) Designation Market research report gives details about the market definition, market drivers, market restraints, market segmentation with respect to product usage and geographical conditions, key developments taking place in the market, competitor analysis, and the research methodology. The analysis and estimations carried out via Breakthrough Therapy (BT) Designation Market research report assist to get the details about the product launches, future products, joint ventures, Market strategy, developments, mergers and acquisitions and effect of the same on sales, Market, promotions, revenue, import, export, and CAGR values.
Learn how the Breakthrough Therapy (BT) Designation Market is evolving—insights, trends, and opportunities await. Download report:
https://www.databridgemarketresearch.com/reports/global-breakthrough-therapy-bt-designation-market
Breakthrough Therapy (BT) Designation Market Introduction
Segments
- Product Type: The Breakthrough Therapy (BT) Designation Market can be segmented based on product type into Drugs and Biologics. The drugs segment is further categorized into small molecules and biologics such as gene and cell therapies.
- Application: This market can also be segmented based on application into Oncology, Infectious Diseases, Autoimmune Disorders, Neurological Disorders, and Others. The oncology segment dominates the market due to the high prevalence of cancer worldwide and the increasing number of BT designations for oncology drugs.
- End Users: The Breakthrough Therapy Designation Market is segmented by end-users into Hospitals, Clinics, Academic & Research Institutes, and Others. The hospitals segment holds a significant share in the market due to the increasing adoption of breakthrough therapies for various diseases.
Market Players
- F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd is a key player in the global Breakthrough Therapy Designation market with a strong portfolio of innovative therapies in oncology, immunology, and other therapeutic areas.
- Novartis AG: Novartis AG is another major player in the market known for its pipeline of breakthrough therapies targeting various diseases including cancer, autoimmune disorders, and rare genetic disorders.
- Pfizer Inc.: Pfizer Inc. is a leading pharmaceutical company with several BT designations for its drugs in oncology, rare diseases, and other therapeutic areas.
- AstraZeneca: AstraZeneca is actively involved in the development of breakthrough therapies for oncology, respiratory diseases, and cardiovascular disorders, contributing significantly to the market growth.
- Johnson & Johnson Services, Inc.: Johnson & Johnson Services, Inc. has a diverse portfolio of breakthrough therapies in areas such as immunology, infectious diseases, and neurology, making it a prominent player in the market.
The global Breakthrough Therapy (BT) Designation Market is witnessing significant growth due to the increasing prevalence of chronic diseases, rising demand for innovative treatment options, and favorable regulatory environment supporting expedited approvals for breakthrough therapies. The market segmentation based on product type, application, and end-users provides insights into the diverse opportunities and challenges present in the market. Key market players such as F. Hoffmann-La Roche Ltd, Novartis AG, Pfizer Inc., AstraZeneca, and Johnson & Johnson Services, Inc. are actively involved in research and development activities to bring novel breakthrough therapies to the market, driving the growth of the BT designation market.
DDDDDThe Breakthrough Therapy Designation market continues to evolve with constant advancements in the pharmaceutical industry and a growing focus on innovative treatment solutions for various diseases. One key trend shaping the market is the increasing collaboration between pharmaceutical companies and research institutes to accelerate the development of breakthrough therapies. This collaborative approach allows for the pooling of resources, expertise, and technologies to bring novel treatments to patients faster, ultimately driving market growth.
Another important aspect influencing the market is the rising interest in personalized medicine, where breakthrough therapies are tailored to target specific genetic markers or disease characteristics in individual patients. This personalized approach not only enhances treatment efficacy but also minimizes adverse effects, thereby creating a significant opportunity for market expansion. Additionally, the integration of advanced technologies such as artificial intelligence and machine learning in drug discovery and development processes is revolutionizing the landscape of breakthrough therapies, enabling more precise and efficient drug design.
Furthermore, the regulatory landscape plays a crucial role in shaping the Breakthrough Therapy Designation market. Regulatory bodies worldwide are increasingly offering expedited pathways and favorable incentives for the development and approval of breakthrough therapies, encouraging pharmaceutical companies to invest more in research and development of innovative treatments. This regulatory support, coupled with a growing demand for effective therapies for complex diseases like cancer, autoimmune disorders, and neurological conditions, is propelling the market forward.
Moreover, the COVID-19 pandemic has highlighted the importance of breakthrough therapies in addressing global health challenges. The rapid development and emergency use authorization of breakthrough vaccines against SARS-CoV-2 have underscored the critical role of innovative treatments in combating emerging infectious diseases. This experience has not only accelerated research efforts in virology and immunology but has also increased public awareness and acceptance of breakthrough therapies, creating a favorable environment for market growth in the coming years.
In conclusion, the Breakthrough Therapy Designation market is poised for significant expansion driven by factors such as collaborative research initiatives, personalized medicine approaches, technological advancements, regulatory support, and the lessons learned from the pandemic. Market players must continue to invest in research and development, forge strategic partnerships, and leverage emerging technologies to capitalize on the growing demand for innovative treatment options across various therapeutic areas. The future of the BT designation market is bright, with opportunities for transformative advancements in healthcare and improved outcomes for patients worldwide.The Breakthrough Therapy Designation market is a dynamic and rapidly evolving sector within the pharmaceutical industry. Key market players such as F. Hoffmann-La Roche Ltd, Novartis AG, Pfizer Inc., AstraZeneca, and Johnson & Johnson Services, Inc. are at the forefront of driving innovation and bringing novel therapies to patients in need. These companies have robust pipelines of breakthrough therapies targeting a wide range of diseases, including oncology, autoimmune disorders, and rare genetic conditions. Their investments in research and development are instrumental in shaping the future of the market and addressing unmet medical needs across various therapeutic areas.
Collaboration between pharmaceutical companies and research institutes plays a pivotal role in accelerating the development of breakthrough therapies. By pooling resources, expertise, and technologies, these partnerships enable faster translation of scientific discoveries into clinical applications, ultimately benefiting patients with innovative treatment options. Moreover, the focus on personalized medicine is transforming the landscape of healthcare by tailoring therapies to individual patients based on their genetic makeup and disease characteristics. This approach not only improves treatment outcomes but also minimizes adverse effects, driving demand for personalized breakthrough therapies.
The integration of advanced technologies such as artificial intelligence and machine learning is revolutionizing drug discovery and development processes in the Breakthrough Therapy Designation market. These technologies enable researchers to analyze vast amounts of data, identify novel drug targets, and design precise therapeutic interventions with higher efficiency and accuracy. As a result, the discovery of breakthrough therapies is becoming more streamlined and cost-effective, leading to a faster pace of innovation and commercialization in the market.
The regulatory landscape also plays a critical role in shaping the growth trajectory of the Breakthrough Therapy Designation market. Regulatory bodies worldwide are simplifying approval pathways and providing incentives for the development of breakthrough therapies to address urgent medical needs. This regulatory support encourages companies to invest in groundbreaking research initiatives and expedite the introduction of innovative treatments to the market. As a result, the market is poised for significant expansion as more breakthrough therapies receive expedited approval and reach patients in a timely manner.
In conclusion, the Breakthrough Therapy Designation market is set for transformative growth driven by collaboration, personalized medicine approaches, technological advancements, and regulatory support. Market players must continue to innovate, collaborate, and harness the power of emerging technologies to meet the evolving needs of patients and healthcare systems worldwide. The future of the market holds great promise for groundbreaking advancements in healthcare and improved patient outcomes across a wide spectrum of diseases.
Gain insights into the firm’s market contribution
https://www.databridgemarketresearch.com/reports/global-breakthrough-therapy-bt-designation-market/companies
Breakthrough Therapy (BT) Designation Market – Analyst-Ready Question Batches
- What is the global market size of the Breakthrough Therapy (BT) Designation Market in 2025?
- What is the expected Breakthrough Therapy (BT) Designation Market value in 2032?
- What segmentation is used in the Breakthrough Therapy (BT) Designation Market analysis?
- Which brands are top contenders in this space?
- What new launches gained the most attention recently?
- Which regions are included in the global Breakthrough Therapy (BT) Designation Market map?
- Which geographic Breakthrough Therapy (BT) Designation Market is growing the fastest?
- Which countries are emerging as high-growth zones?
- What region leads in terms of profit contribution?
- What are the key challenges the Breakthrough Therapy (BT) Designation Market faces?
Browse More Reports:
Middle East and Africa Gummies and Jellies Market
Europe Recovered Carbon Black (rCB) Market
North America Popping Boba Juice Balls Market
Middle East and Africa Colorectal Cancer Diagnostics Market
Europe Web Hosting Services Market
Asia Pacific Biochar Market
North America Biochar Market
Global Agricultural Wastewater Treatment Market
Global Aluminium-extruded Products Market
Global Automotive Piston Pin Market
Global Bone Marrow Biopsy Market
Global Camel Dairy Market
Global Carnauba Wax Market
Global Cattle Feed and Feed Additives Market
Global Cigarette Rolling Paper Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"


