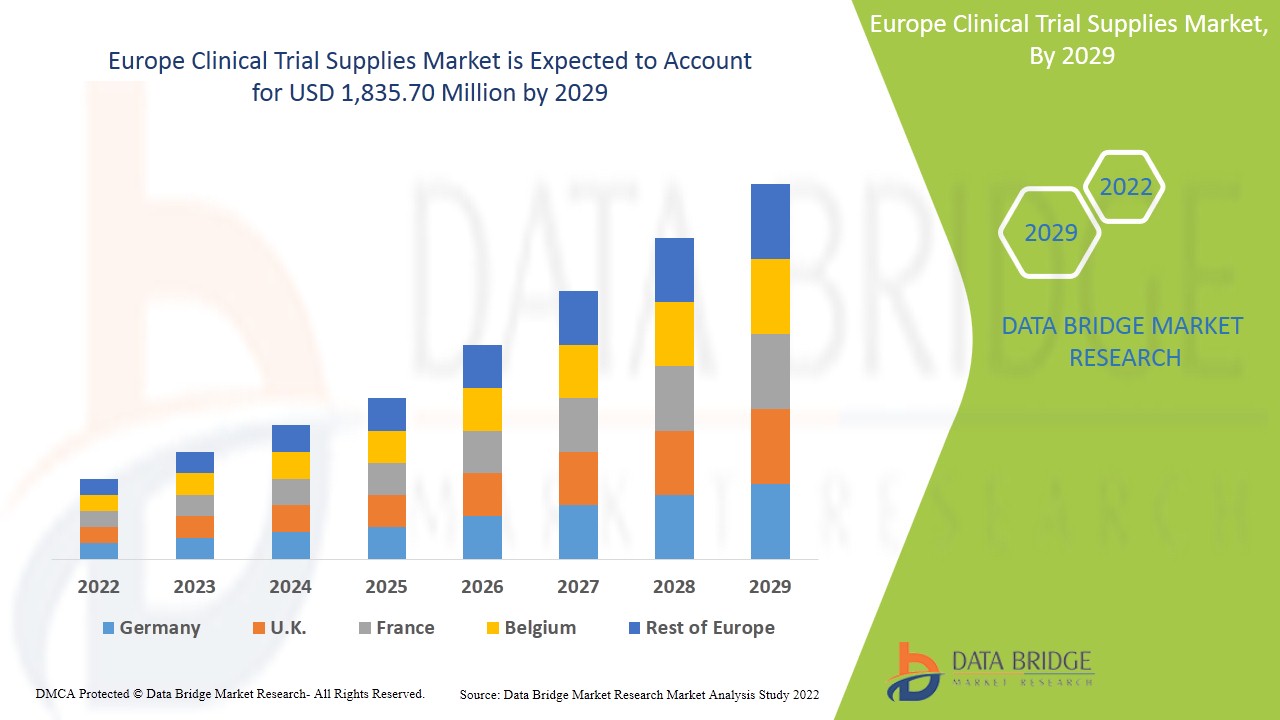
"Executive Summary Europe Clinical Trial Supplies Market Trends: Share, Size, and Future Forecast
Data Bridge Market Research analyses that the market is growing with a CAGR of 7.8% in the forecast period of 2022 to 2029 and is expected to reach USD 1,835.70 million by 2029.
Being an excellent market research report, Europe Clinical Trial Supplies Market report serves as a backbone for the business when it is about thriving in the competition. The report is all-embracing global market research report and it identifies, analyses, and estimates the emerging trends along with major drivers, challenges and opportunities in the Europe Clinical Trial Supplies Market industry along with the analysis of retailers, geographical regions, types, and applications. This industry report studies wide-ranging industry analysis with exact estimates and forecasts that provides complete research solutions with maximum industry clarity. Thus, Europe Clinical Trial Supplies Market report is the best solution for businesses if they want to stay ahead of the competition in today's rapidly moving business landscape.
The finest Europe Clinical Trial Supplies Market analysis report provides estimations about the growth rate and the market value based on market dynamics and growth inducing factors. Businesses can get current as well as upcoming technical and financial details of the industry to 2030 with this market research report. The report is mainly distributed to the users in the form of PDF or spreadsheet or PPT (if asked by client). The report also mentions CAGR value fluctuations during the forecast period of 2023-2030 for the market. The winning Europe Clinical Trial Supplies Market report has been formulated with the best-practice models, comprehensive market analysis and research methodologies so that clients attain perfect market segmentation and insights.
Examine detailed statistics, forecasts, and expert analysis in our Europe Clinical Trial Supplies Market report. Download now:
https://www.databridgemarketresearch.com/reports/europe-clinical-trial-supplies-market
Europe Clinical Trial Supplies Sector Overview
Segments
- Product Type: The Europe clinical trial supplies market can be segmented on the basis of product type into manufacturing services,clinical trial manufacturing, logistics and distribution services, commercialization services, and others. Manufacturing services would account for a significant share due to the increasing demand for outsourcing manufacturing activities to streamline the process and ensure quality.
- Pharmaceutical and biopharmaceutical companies: These companies have a significant impact on the clinical trial supplies market in Europe. With the increasing number of clinical trials being conducted by these companies, the demand for clinical trial supplies is also on the rise.
- Types of Phases: Clinical trials are usually conducted in multiple phases, such as Phase I, Phase II, Phase III, and Phase IV. Each phase requires different types of supplies and services, thereby creating distinct segments within the market.
Market Players
- Thermo Fisher Scientific Inc.: Thermo Fisher Scientific Inc. is one of the leading players in the Europe clinical trial supplies market, with a wide range of products and services catering to the pharmaceutical and biopharmaceutical companies.
- Catalent, Inc.: Catalent, Inc. is another key player in the market, offering comprehensive solutions for clinical trial supplies, including manufacturing, packaging, and distribution services.
- Sharp Clinical Services: Sharp Clinical Services is known for its expertise in clinical trial supplies management, providing specialized packaging and distribution services to ensure the integrity of the supplies throughout the trial process.
The Europe clinical trial supplies market is witnessing significant growth with the increasing number of clinical trials being conducted in the region. Factors such as technological advancements, growing investments in research and development activities, and the presence of key market players are driving the market forward. Pharmaceutical and biopharmaceutical companies play a crucial role in shaping the market dynamics by fueling the demand for various clinical trial supplies and services. Additionally, the segmentation based on product type and phases helps in understanding the specific requirements of different stages of clinical trials, thereby enabling companies to tailor their offerings to meet the evolving needs of the market.
Overall, the Europe clinical trial supplies market is poised for further growth, with advancements in healthcare infrastructure, increasing focus on precision medicine, and rising investments in clinical research activities. The market players mentioned above, along with other prominent companies, are likely to continue innovating and expanding their product portfolios to stay competitive in this dynamic market landscape.
The Europe clinical trial supplies market continues to evolve with various factors influencing its growth trajectory. One key aspect that can provide new insights into the market is the increasing emphasis on personalized medicine and its impact on clinical trials. Personalized medicine aims to tailor medical treatment to the individual characteristics of each patient, including their genetic makeup, lifestyle, and environmental factors. This trend is driving the demand for more targeted and specific clinical trials that require specialized supplies and services to accommodate the unique needs of individual patients.
Another emerging trend in the Europe clinical trial supplies market is the integration of digital technologies and data analytics in clinical research. The use of digital platforms, wearable devices, and real-time data monitoring tools is revolutionizing the way clinical trials are conducted, leading to more efficient and accurate results. Market players are increasingly investing in technology-driven solutions to enhance the delivery of clinical trial supplies and improve overall trial management processes.
Furthermore, the growing focus on sustainability and environmentally friendly practices is influencing the choices made by pharmaceutical and biopharmaceutical companies when selecting clinical trial suppliers. Companies that prioritize sustainable sourcing, packaging, and distribution practices are gaining a competitive edge in the market and are likely to see increased demand for their services. This shift towards sustainability is not only driven by regulatory requirements but also by the changing preferences of consumers and stakeholders who are increasingly conscious of environmental impact.
Additionally, the COVID-19 pandemic has had a significant impact on the Europe clinical trial supplies market, leading to disruptions in trial timelines, supply chain challenges, and increased emphasis on remote monitoring and virtual clinical trials. Market players have had to quickly adapt to the new normal brought about by the pandemic, resulting in greater collaboration, innovation, and flexibility in the delivery of clinical trial supplies. These changes are likely to have lasting effects on the market dynamics, shaping the future of clinical research and trial management in the region.
In conclusion, the Europe clinical trial supplies market is a dynamic and rapidly evolving industry influenced by various trends and factors. From the rise of personalized medicine to the integration of digital technologies and the increasing focus on sustainability, market players need to stay agile and innovative to capitalize on emerging opportunities and meet the evolving needs of the healthcare sector. The market's future growth will be driven by continued advancements in technology, changing patient demographics, and the shifting regulatory landscape, shaping a more interconnected and data-driven approach to clinical trials in Europe.The Europe clinical trial supplies market is a complex and multifaceted industry that is continuously evolving to adapt to changing trends and demands. One key aspect shaping the market is the increasing emphasis on personalized medicine. The shift towards personalized medicine is driving the need for more targeted and specific clinical trials, requiring specialized supplies and services to cater to individual patient characteristics. This trend is expected to drive the demand for innovative solutions and customized offerings from market players to support the unique requirements of personalized medicine-driven clinical trials.
Another important trend influencing the Europe clinical trial supplies market is the integration of digital technologies and data analytics in clinical research. The adoption of digital platforms, wearable devices, and real-time data monitoring tools is revolutionizing the way clinical trials are conducted, leading to enhanced efficiency and accuracy in trial outcomes. Market players are proactively investing in technology-driven solutions to improve the delivery of clinical trial supplies and optimize trial management processes. This trend is crucial for meeting the growing demand for data-driven insights and improving overall trial outcomes in the European market.
Moreover, the market is witnessing a notable shift towards sustainability and environmentally friendly practices among pharmaceutical and biopharmaceutical companies. Companies that prioritize sustainable sourcing, packaging, and distribution practices are gaining a competitive edge in the market and are likely to experience increased demand for their services. The focus on sustainability is not only driven by regulatory requirements but also by the changing preferences of consumers and stakeholders who are placing greater emphasis on environmental consciousness. Market players that align with sustainable practices are well-positioned to capitalize on the growing awareness and demand for environmentally friendly clinical trial supplies and services in Europe.
Additionally, the COVID-19 pandemic has significantly impacted the Europe clinical trial supplies market, leading to disruptions in trial timelines, supply chain challenges, and a heightened focus on remote monitoring and virtual clinical trials. Market players have had to swiftly adapt to the new realities brought about by the pandemic, fostering greater collaboration, innovation, and flexibility in supply chain management and trial operations. These adaptations are expected to have long-lasting effects on the market landscape, driving further advancements in virtual trial technologies, remote monitoring solutions, and flexible supply chain strategies to meet the evolving needs of clinical research in the post-pandemic era.
In conclusion, the Europe clinical trial supplies market is undergoing profound changes driven by the increasing emphasis on personalized medicine, digital transformation, sustainability practices, and the lasting impact of the COVID-19 pandemic. Market players must remain agile, innovative, and responsive to emerging trends to capitalize on new opportunities and address the evolving demands of the healthcare sector. Embracing technology, sustainable practices, and adaptive strategies will be essential for driving growth and competitiveness in the dynamic landscape of clinical trial supplies in Europe.
View company-specific share within the sector
https://www.databridgemarketresearch.com/reports/europe-clinical-trial-supplies-market/companies
Strategic Question Sets for In-Depth Europe Clinical Trial Supplies Market Analysis
- What is the current revenue pool of the Europe Clinical Trial Supplies Market?
- How is the annualized growth expected to trend?
- What functional segments are analyzed in the Europe Clinical Trial Supplies Market report?
- Who are the companies with the most aggressive growth plans?
- What recent upgrades have been introduced to leading products?
- What countries are major contributors to global Europe Clinical Trial Supplies Market demand?
- What region is experiencing structural transformation?
- Which countries are leading exporters of related products?
- Where is product acceptance highest?
- What are the cross-industry trends influencing growth for Europe Clinical Trial Supplies Market?
Browse More Reports:
Asia-Pacific Obsessive-Compulsive Disorder (OCD) Drugs MarketNorth America Obsessive-Compulsive Disorder (OCD) Drugs MarketMiddle East and Africa Obsessive-Compulsive Disorder (OCD) Drugs MarketEurope Obsessive-Compulsive Disorder (OCD) Drugs MarketU.A.E. Balsamic Vinegar MarketAsia-Pacific Balsamic Vinegar MarketNorth America Balsamic Vinegar MarketMiddle East and Africa Balsamic Vinegar MarketEurope Balsamic Vinegar MarketEurope Telecom Expense Management MarketMiddle East and Africa Telecom Expense Management MarketMiddle East and Africa Automotive Sensors MarketNorth America Health Screening MarketMiddle East and Africa Health Screening MarketMiddle East and Africa Operating Room Equipment Supplies Market
U.S Multiple Myeloma Diagnostic Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"